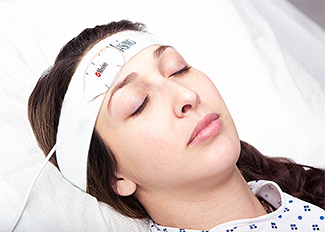
(English) Single-patient-use sensor allows clinicians to monitor patients
Din păcate acest articol este disponibil doar în Engleză Americană. For the sake of viewer convenience, the content is shown below in the alternative language. You may click the link to switch the active language. Masimo (NASDAQ: MASI) announced today FDA 510(k) clearance for the TFA-1™ Single-Patient-Use Adhesive Forehead Sensor. This single-patient-use sensor allows clinicians Citește mai mult despre(English) Single-patient-use sensor allows clinicians to monitor patients[…]