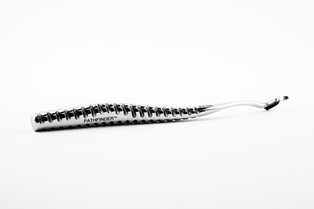
A 3D printed surgical tool may be a medical breakthrough high-performance athletes have been waiting for. DanaMed™ Inc.’s Pathfinder™ ACL Guide is a biocompatible surgical device enabling surgeons to better reconstruct partially or fully torn anterior cruciate ligaments (ACL) and reduce the risk of re-tearing. Stratasys Direct Manufacturing, one of the largest providers of additive (3D printing) and conventional manufacturing services in North America, builds the metal tool using Direct Metal Laser Sintering (DMLS™) technology.
 „Pathfinder illustrates how 3D printing is uniquely capable of enabling breakthroughs in medical technology that otherwise would not be possible,” said John Self, project engineer at Stratasys Direct Manufacturing. „And by offering DanaMed 97 percent cost savings over conventional manufacturing methods, 3D printing has demonstrated its business value in bringing complex, high-quality parts to market.”
„Pathfinder illustrates how 3D printing is uniquely capable of enabling breakthroughs in medical technology that otherwise would not be possible,” said John Self, project engineer at Stratasys Direct Manufacturing. „And by offering DanaMed 97 percent cost savings over conventional manufacturing methods, 3D printing has demonstrated its business value in bringing complex, high-quality parts to market.”
Dr. Dana Piasecki, an orthopedic surgeon at OrthoCarolina Sports Medicine, in Charlotte, N.C., developed the Pathfinder System, comprised of the Pathfinder ACL Guide and Guide Pins, after experimenting with surgical techniques that would improve graft positioning. The key, he discovered, is using a surgical tool specially shaped to match the anatomy of the knee. After refining the design using Fused Deposition Modeling™ (FDM®), Dr. Piaseckiand DanaMed Inc. needed a manufacturing process that could efficiently produce the complex surgical instrument at an affordable price and provide the freedom necessary to make design changes on the fly. Stratasys DirectManufacturing met these requirements by using DMLS.
The Pathfinders are printed with Inconel 718 material, which achieve the necessary biocompatibility, surface finish, oil resistance and mechanical requirements. After extensive testing, the Pathfinder System was registered with the FDA as a Class 1 Medical Device. Pathfinders are now on the market and being used by orthopedic surgeons across the country.
With a 95 % success rate of anchoring grafts in their native ACL locations, DanaMed’s Pathfinder System is a potential game-changer for ACL repair surgeries. Anchoring grafts in this way allows repaired ACLs to handle the same stress as a natural ACL once could. Other methods are more difficult to perform and can increase both the potential for surgical complications and risk of reinjuring the knee.
Like DanaMed, more and more companies are manufacturing metal parts via 3D printing. In fact, a recent survey sponsored by Stratasys DirectManufacturing, „3D Printing’s Imminent Impact on Manufacturing,” found additive metal usage in the U.S. is expected to nearly double over the next three years. To meet the mounting market demand for metals, Stratasys Direct Manufacturing has nearly tripled its additive metals capacity over the past 18 months.
Source: Stratasys